Laura E. Ichikawa, MS
Biography
With more than 25 years at Kaiser Permanente Washington Health Research Institute (KPWHRI), Laura Ichikawa, MS, is known for her analytical skills, attention to detail regarding study design and data management, and knowledge in data visualization. Her experience includes observational studies, randomized controlled trials, case-control studies, and multisite analyses.
Laura is primarily focused on cancer research and women’s health. She’s has been involved with the Statistical Coordinating Center for the Breast Cancer Surveillance Consortium (BCSC), funded by the National Cancer Institute. She leads both data management activities and statistical analyses for the BCSC, where much of her work has been evaluating mammography performance for both screening and surveillance while also examining use of screening MRI (magnetic resonance imaging) and ultrasound. Laura also participated in collaborative research through the Cancer Research Network, including a widely publicized 2004 study that linked not having screening mammograms to late-stage breast cancer and, more recently, the Population-based Research to Optimize the Screening Process (PROSPR) project focusing on colorectal cancer screening. She is excited to continue her work in cancer research, including in screening, treatment, surveillance, and survival.
Laura’s earlier research with retired KPWHRI Senior Investigator Delia Scholes, PhD, included studies examining bone loss associated with the injectable hormonal contraceptive Depo-Provera in 2002 and 2005 and oral contraceptives in 2011.
Laura is a member of the American Statistical Association and its Puget Sound chapter.
Research interests and experience
-
Biostatistics
Longitudinal data analysis
-
Cancer
Biostatistics; breast cancer
-
Women's Health
Biostatistics; hormonal contraception and bone density
Recent publications
Su YR, Buist DSM, Lee JM, Ichikawa L, Miglioretti DL, Bowles EJA, Wernli KJ, Kerlikowske K, Tosteson A, Lowry KP, Henderson LM, Sprague BL, Hubbard RA Performance of statistical and machine learning risk prediction models for surveillance benefits and failures in breast cancer survivors 2023 Apr 3;32(4):561-571. doi: 10.1158/1055-9965.EPI-22-0677. Epub 2023-01-25. PubMed
Anthony MS, Zhou X, Schoendorf J, Reed SD, Getahun D, Armstrong MA, Gatz J, Peipert JF, Raine-Bennett T, Fassett MJ, Saltus CW, Ritchey ME, Ichikawa L, Shi JM, Alabaster A, Wahdan Y, Wang J, Xie F, Merchant M, Hunter S, Chiu VY, Postlethwaite D, Rothman KJ, Im TM, Chillemi G, Takhar HS, Asiimwe A, Pisa F Demographic, Reproductive, and Medical Risk Factors for Intrauterine Device Expulsion 2022 Dec;140(6):1017-1030. doi: 10.1097/AOG.0000000000005000. Epub 2022-11-02. PubMed
Pocobelli G, Ichikawa L, Yu O, Green BB, Meyers K, Gray R, Shea M, Chubak J Validation of international classification of diseases, tenth revision, clinical modification diagnosis codes for heart failure subtypes 2022 Sep;31(9):992-997. doi: 10.1002/pds.5489. Epub 2022-06-07. PubMed
Gatz JL, Armstrong MA, Postlethwaite D, Raine-Bennett T, Chillemi G, Alabaster A, Merchant M, Reed SD, Ichikawa L, Getahun D, Fassett MJ, Shi JM, Xie F, Chiu VY, Im TM, Takhar HS, Wang J, Saltus CW, Ritchey ME, Asiimwe A, Pisa F, Schoendorf J, Wahdan Y, Zhou X, Hunter S, Anthony MS, Peipert JF Association Between Intrauterine Device Type and Risk of Perforation and Device Expulsion: Results From the APEX-IUD Study 2022 Jul;227(1):57.e1-57.e13. doi: 10.1016/j.ajog.2022.03.062. Epub 2022-04-05. PubMed
Getahun D, Fassett MJ, Gatz J, Armstrong MA, Peipert JF, Raine-Bennett T, Reed SD, Zhou X, Schoendorf J, Postlethwaite D, Shi JM, Saltus CW, Wang J, Xie F, Chiu VY, Merchant M, Alabaster A, Ichikawa LE, Hunter S, Im TM, Takhar HS, Ritchey ME, Chillemi G, Pisa F, Asiimwe A, Anthony MS Association Between Menorrhagia and Risk of Intrauterine Device-Related Uterine Perforation and Device Expulsion: Results from the APEX-IUD Study 2022 Jul;227(1):59.e1-59.e9. doi: 10.1016/j.ajog.2022.03.025. Epub 2022-03-12. PubMed
Reed SD, Zhou X, Ichikawa L, Gatz JL, Peipert JF, Armstrong MA, Raine-Bennett T, Getahun D, Fassett MJ, Postlethwaite DA, Shi JM, Asiimwe A, Pisa F, Schoendorf J, Saltus CW, Anthony MS, APEX-IUD study team Intrauterine device-related uterine perforation incidence and risk (APEX-IUD): a large multisite cohort study 2022 Jun 4;399(10341):2103-2112. doi: 10.1016/S0140-6736(22)00015-0. PubMed
Armstrong MA, Raine-Bennett T, Reed SD, Gatz J, Getahun D, Schoendorf J, Postlethwaite D, Fassett MJ, Peipert JF, Saltus CW, Merchant M, Alabaster A, Zhou X, Ichikawa L, Shi JM, Chiu VY, Xie F, Hunter S, Wang J, Ritchey ME, Chillemi G, Im TM, Takhar HS, Pisa F, Asiimwe A, Anthony MS Association of the Timing of Postpartum Intrauterine Device Insertion and Breastfeeding With Risks of Intrauterine Device Expulsion 2022 Feb;5(2):e2148474. doi: 10.1001/jamanetworkopen.2021.48474. Epub 2022-02-01. PubMed
Lee JM, Ichikawa LE, Wernli KJ, Bowles E, Specht JM, Kerlikowske K, Miglioretti DL, Lowry KP, Tosteson ANA, Stout NK, Houssami N, Onega T, Buist DSM Digital Mammography and Breast Tomosynthesis Performance in Women with a Personal History of Breast Cancer, 2007-2016 2021 Aug;300(2):290-300. doi: 10.1148/radiol.2021204581. Epub 2021-05-18. PubMed
Buist DSM, Ichikawa L, Wernli KJ, Lee CI, Henderson LM, Kerlikowske K, Bowles EJA, Miglioretti DL, Specht J, Rauscher GH, Sprague BL, Onega T, Lee JM Facility Variability in Examination Indication Among Women With Prior Breast Cancer: Implications and the Need for Standardization 2020 Jun;17(6):755-764. doi: 10.1016/j.jacr.2019.12.020. Epub 2020-01-28. PubMed
D Sullivan M, Boudreau D, Ichikawa L, Cronkite D, Albertson-Junkans L, Salgado G, VonKorff M, Carrell DS Primary Care Opioid Taper Plans Are Associated with Sustained Opioid Dose Reduction 2020 Mar;35(3):687-695. doi: 10.1007/s11606-019-05445-1. Epub 2020-01-06. PubMed
Research

Study measures risks of screening colonoscopies for older adults
The findings can help guide colorectal cancer screening decisions later in life.
Research
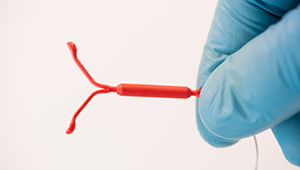
IUD perforation: Rare but important to know about
New study results in more precise language in the federally mandated warning about this possibility.
cancer research

Using breast MRI after cancer may lead to unneeded biopsies
A Kaiser Permanente-led BCSC study is among the largest ever to evaluate adding MRI surveillance for breast cancer survivors.
cancer screening

No need to follow up simple ovarian cysts
New KPWHRI study shows that women with simple cysts are not at increased risk of ovarian cancer.



