Study evaluates biomarker criteria for Alzheimer’s risk
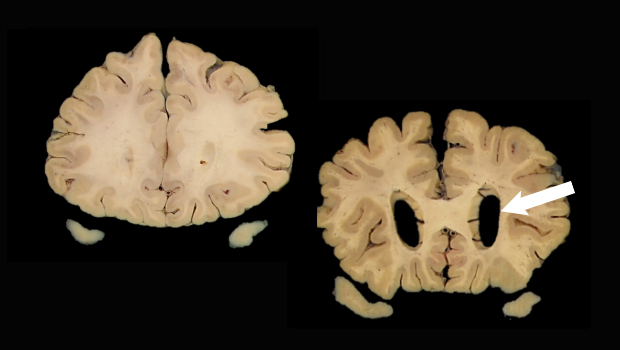
Scans show a normal brain (left) and a brain with enlarged ventricles (arrow) suggesting neurodegeneration, a biomarker for Alzheimer’s disease. Images: Dr. Caitlin Latimer, UW Department of Laboratory Medicine and Pathology
One-third of people classified as ‘highest risk’ may not develop Alzheimer’s disease, study suggests
A new study co-authored by researchers at Kaiser Permanente Washington Health Research Institute suggests one-third of people classified as having the highest risk for dementia by a biomarker research tool — the AT(N) framework — will not develop dementia in their lifetime.
“[This research] highlights the importance of studying not just what we are today calling ‘Alzheimer’s disease’ but also the factors that seem to make people resilient or able to resist cognitive decline and dementia, which are so often seen as inevitable features of aging,” said Eric Larson, MD, MPH, senior investigator and former vice president for research and health care innovation at KPWHRI, who was a senior author on the study.
Biomarkers: some background
One of the biggest challenges in dementia research is to identify people who are at risk of developing Alzheimer’s disease. Biomarkers could be used to screen people so they might be helped before they develop the disease.
Researchers have focused primarily on 3 such biomarkers. Two are Alzheimer’s-related proteins called amyloid and tau, which can be detected in cerebral spinal fluid or by specialized positron emission tomography (PET) scans. The third marker, brain atrophy, can be seen with computerized tomography and MRI scans.
The AT(N) framework is a research tool based on these biomarkers — A for amyloid, T for tau, and (N) for neurodegeneration or atrophy. The National Institute on Aging and the Alzheimer’s Association (NIA-AA) have endorsed this tool in their efforts to develop a framework to assess an asymptomatic person’s risk of developing dementia.
In the new study — published in Alzheimer's & Dementia: The Journal of the Alzheimer's Association — researchers evaluated this framework by analyzing data from the autopsies of people who participated in the long-running Adult Changes in Thought (ACT) study. Bridget Teevan Burke, a research fellow at KPWHRI, was the lead author of the new study. Joining Dr. Larson as a senior author was Paul Crane, MD, MPH, professor of medicine at the University of Washington School of Medicine, Division of General Internal Medicine, and an affiliate researcher with KPWHRI. Drs. Larson and Crane are also the principal investigators of the ACT study.
Analyzing ACT autopsy data
The ACT study, which started in the 1990s, has followed more than 5,500 older volunteers to identify new cases of dementia. About a third of the volunteers allowed their brains to be studied after death.
Since Alzheimer’s often progresses slowly, the ACT participant autopsy results for amyloid, tau, and neurodegeneration were used to approximate AT(N) classification 5 years before death. The researchers considered evaluations of the participants before their death to see whether the proxy AT(N) categorization would predict dementia development in those 5 years.
The AT(N) framework uses 8 different biomarker profiles, ranging from none of the biomarkers present, designated A-T-(N)-, to profiles where all biomarkers are present, designated A+T+(N)+, as well as all the possible combinations, such as A+T+(N)-, indicating amyloid and tau are present but no neurodegeneration.
The researchers found that 67% of those with the proxy A+T+(N)+ profile developed dementia in the next 5 years. But 33% did not.
“A+T+(N)+ is supposed to be the highest-risk group,” said Dr. Crane. “They have high levels of amyloid, high levels of tau, and have atrophy. Even in that group, a third never developed dementia.”
The findings, if validated with AT(N) biomarkers, suggest that any drug trial using the AT(N) framework will require many more participants to be screened to enroll enough people to achieve statistically solid results, Dr. Crane said. Because PET scans used to detect amyloid and tau cost thousands of dollars, such studies would be even more expensive.
Dr. Caitlin Latimer, a neuropathologist at the UW and a co-author on the paper, noted that “although this study was limited to assessments of amyloid, tau, and neurodegeneration at autopsy, it serves as a reminder that these components are just one part of the complicated puzzle that is age-related cognitive decline. The AT(N) framework is valuable because it places living people on the spectrum of AD neuropathologic change, which will allow us to better study the concept of resilience to this pathology and encourage the development of additional biomarkers necessary for the accurate prediction of who will go on to develop dementia.”
Dr. Larson concluded, “This paper is important and was possible because of the longtime commitments of ACT subjects and their families, an enduring partnership between Kaiser Permanente Washington (formerly Group Health) and the University of Washington, and decades of funding from the National Institute on Aging.”
The study was funded by the National Institutes of Health, National Institute on Aging (U01AG006781, P50AG005136, 1K08AG065426-01), and the Nancy and Buster Alvord Endowment.
This story was adapted from a news release written by Michael McCarthy for the University of Washington School of Medicine.
aging and geriatrics
The ACT Study: Looking toward the future
At 25 years, the Adult Changes in Thought Study is committed to science, collaboration, and jokes, writes Erin Bowles
Aging and geriatrics
ACT study: Long-running study of aging examines changes in Kaiser Permanente patients over time
Drs. Larson and Crane co-lead Kaiser Permanente-University of Washington collaboration learning how to promote healthy aging


