COVID-19 vaccine well tolerated, generates immune response
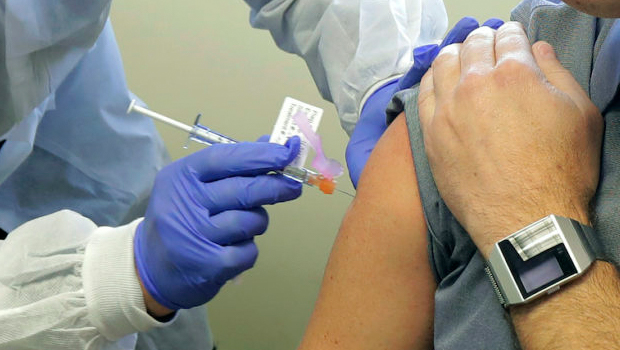
A pharmacist gives the investigational COVID-19 vaccine to a volunteer in the NIH-supported clinical trial in Seattle on March 16. Credit: Ted S. Warren / AP Photos
National Institute of Allergy and Infectious Diseases-sponsored phase 1 trial tested mRNA vaccine
SEATTLE — An investigational vaccine designed to protect against COVID-19 was generally well tolerated and prompted neutralizing activity in healthy adults, according to interim results published today in the New England Journal of Medicine. The lead author of the study, detailed in “A SARS-CoV-2 mRNA Vaccine — Preliminary Report,” is Lisa A. Jackson, MD, MPH, a senior investigator at Kaiser Permanente Washington Health Research Institute in Seattle.
"The world urgently needs vaccines to protect against COVID-19,” Dr. Jackson said. “We are glad to be able to contribute to these efforts by initiating the first clinical trial of a COVID-19 vaccine, which was developed, produced, and put into a first-in-human clinical trial in record time.”
The ongoing phase 1 trial is supported by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. The experimental vaccine is being co-developed by researchers at NIAID and at Moderna Inc. of Cambridge, Massachusetts. Manufactured by Moderna, mRNA-1273 is designed to induce neutralizing activity to block the virus from binding to and entering human cells.
The first study vaccination was administered on March 16 at Kaiser Permanente Washington Health Research Institute. The interim report details the findings from the first 45 participants ages 18 to 55 years enrolled at the study sites in Seattle and Emory University in Atlanta through 57 days after their first vaccination. Three groups of 15 volunteers received 2 intramuscular injections, 28 days apart, of either 25, 100, or 250 micrograms of the investigational vaccine.
In April, the trial was expanded to enroll adults older than 55 years; it now has 120 participants. However, the newly published results cover the 18- to 55-year age group only.
Regarding safety, no serious adverse events were reported. More than half of the participants reported symptoms including fatigue, headache, chills, muscle aches, or pain at the injection site. Symptoms were more common following the second vaccination and in those who received the highest vaccine dose. Data on side effects and immune responses at various vaccine dosages informed the doses used or planned for use in the phase 2 and 3 clinical trials of the investigational vaccine.
The interim analysis reports on the levels of vaccine-induced neutralizing activity—through days 43 to 57—after the second injection. Two doses of vaccine prompted high levels of neutralizing activity that were above the average values seen in convalescent serum obtained from people with confirmed cases of COVID-19 disease.
COVID-19 vaccine registries
A phase 2 clinical trial of mRNA-1273, sponsored by Moderna, began enrollment in late May. Plans are underway to launch a phase 3 efficacy trial in July 2020.
Kaiser Permanente Washington’s vaccine research group has a registry of Seattle-area people who want to be considered to participate in possible trials of investigational vaccines for COVID-19 at Kaiser Permanente Washington in Seattle: corona.kpwashingtonresearch.org.
The COVID-19 Prevention Trials Network has a volunteer screening registry of people nationwide who are interested in participating in a broader range of vaccines and other preventive treatments at various research facilities in Seattle and around the country: coronaviruspreventionnetwork.org.
Additional information about the phase 1 trial design is available at clinicaltrials.gov using the identifier NCT04283461. This trial was supported in part by NIAID grants UM1AI148373 (Kaiser Permanente Washington), UM1AI148576 (Emory University), and UM1AI148684 (Infectious Diseases Clinical Research Consortium). The Coalition for Epidemic Preparedness Innovations (CEPI) provided funding for the manufacture of mRNA-1273 phase 1 material.
Read more about Kaiser Permanente’s research on vaccines.
About the National Institute of Allergy and Infectious Diseases
NIAID conducts and supports research — at NIH, throughout the United States, and worldwide — to study the causes of infectious and immune-mediated diseases and to develop better means of preventing, diagnosing, and treating these illnesses. News releases, fact sheets, and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health
NIH, the nation's medical research agency, includes 27 institutes and centers and is a component of the Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit the NIH website.
About Kaiser Permanente Washington Health Research Institute
Kaiser Permanente Washington Health Research Institute (KPWHRI) improves the health and health care of Kaiser Permanente members and the public. The Institute has conducted nonproprietary public-interest research on preventing, diagnosing, and treating major health problems since 1983. Government and private research grants provide our main funding. Follow KPWHRI research on Twitter, Facebook, LinkedIn, or YouTube. For more information, go to: www.kpwashingtonresearch.org.
research
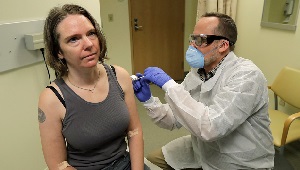
Kaiser Permanente launches first coronavirus vaccine trial
On March 16, 4 volunteers received an injection of an mRNA vaccine for COVID-19 in an NIH-funded trial in Seattle.
Research
COVID-19 pandemic research at KPWHRI
Having long tracked infectious diseases and tested vaccines, KPWHRI now focuses on the novel coronavirus.



