Mailing home HPV tests may provide alternative to Pap screening
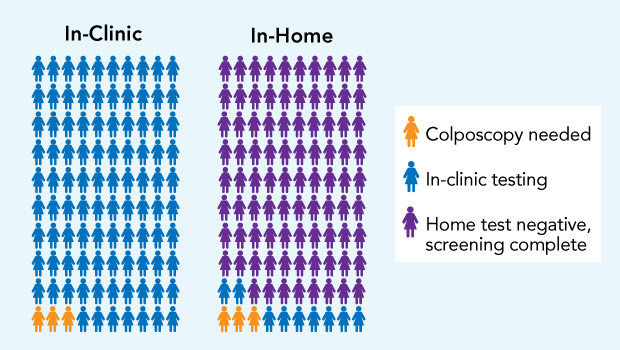
In the HOME trial, for every 100 women who got in-clinic Pap tests with usual care (left), 97 could be resolved with cervical samples taken at that test (blue), and 3 needed another in-clinic visit for colposcopy, a diagnostic procedure to examine the cervix with a scope (yellow). For every 100 mailed in-home HPV tests (right), 88 women had normal results (purple), without needing a clinic visit, and 12 needed in-clinic testing: 9 Pap tests (blue), and 3 colposcopies (yellow).
Dr. Diana Buist and team reflect on HOME trial showing 50 percent screening boost in underscreened women
The HOME (Home-based Options to Make cervical cancer screening Easy) trial is the first—and only—randomized trial to study mailed HPV test kits in a real-world U.S. health system: Kaiser Permanente Washington. JAMA Network Open just published its main results: “Effect of Mailed Human Papillomavirus Test Kits vs Usual Care Reminders on Cervical Cancer Screening Uptake, Precancer Detection, and Treatment: A Randomized Clinical Trial.” Some of the paper’s coauthors discuss it, including Diana S.M. Buist, PhD, Kaiser Permanente Washington’s lead investigator on the trial; and Rachel L. Winer, PhD, first author and principal investigator:
Why does this work matter?
Dr. Buist: Cervical cancer screening has been one of the most effective cancer tests for saving lives. Cancer of the cervix can be largely prevented with regular screening—usually using Pap (Papanicolaou) tests by clinicians. But one in four American women delay screening for cervical cancer—or don’t get screened at all. And half of the 12,000 cervical cancers diagnosed each year in the US are in these underscreened women, whose numbers are actually rising now. Pap tests look for abnormal-looking cells. HPV tests are more direct, because they detect the high-risk strains of human papillomavirus that can cause cervical cancer. Sometimes, HPV remains inactive in the cells and can be re-activated later. That’s why women under age 65 should get screened regularly even if they’ve been with the same partner or have not been sexually active for many years.
What did you find?
Rachel Winer: We found that sending home-based HPV testing is feasible, and it’s an effective strategy for encouraging hard-to-reach women to get screened for cervical cancer.
Dr. Buist: Women who received a kit mailed to their home were 50 percent more likely to be screened compared to those who received usual care: annual patient reminders and outreach from primary care clinics. We also found that women got screened more quickly if they were in the group who received the mailing.
How did you conduct the trial?
Dr. Buist: We enrolled nearly 20,000 women at Kaiser Permanente Washington who were age 30-64 and overdue for cervical cancer screening, and we randomly assigned them to receive either usual care or that plus a mailed HPV test kit and instructions for them to take a sample at home. Women in the second group could opt to complete the self-collection kit and return their samples in the mail to our laboratory at Kaiser Permanente—or to be screened in the clinic like the usual-care group.
Why explore this alternative to Pap screening?
Diana Miglioretti: The flexibility of being able to do this cancer screening test at home is very exciting for busy women who may lack the time, transportation, child care, or inclination to visit their physician for Pap screening.
Dr. Buist: Many women aren’t thrilled about going in for a Pap test. They may be overdue for screening because they dislike the procedure or simply don’t have the time to attend in-clinic screening. HPV testing in the privacy and comfort of home promises to put care in the hands of women, remove barriers to care, and increase access and affordability.
Kilian Kimbel: When I talked with women in the trial, some were reluctant to try this new screening method. But many others said the in-home testing was much easier than Pap screening—and helped them feel more in charge of their own health.
How did the trial happen?
Dr. Buist: The trial shows how much science is a team sport. It all started with Rachel Winer’s idea for what became the HOME trial. Her expertise in HPV and cervical cancer screening, and mine in cancer screening and research embedded in a delivery system, were key ingredients to getting funding and executing this important trial.
Dr. Winer: Many other countries, including Australia and the Netherlands, include the option of home HPV screening for underscreened women. But Kaiser Permanente Washington is the only place in the United States that has done this work. The opportunity to conduct a pragmatic trial embedded within the Kaiser Permanente Washington care-delivery system has been very rewarding.
Dr. Buist: Implementing the trial depended on our clinical colleagues in the Kaiser Permanente Washington delivery system. We nominated them for the 2019 Birnbaum Award, and we’re delighted that they won it.
Dr. Chris Thayer: This trial is an example of the type of innovative thinking that KPWHRI brings to Kaiser Permanente and to our members.
Dr. Buist: Even with enthusiastic support from the care-delivery system, conducting this trial was hard—probably the most challenging of my career to date. But the challenges were surmountable, and the promise of helping to make the option of home testing available to women is worth it.
What were the challenges along the way?
Dr. Buist: U.S. guidelines for cervical cancer are complex and keep evolving with new evidence. It wasn’t until 2018 that the U.S. Preventive Task Force changed its cervical cancer screening recommendations to include the option of HPV screening, without Pap testing, every 5 years for women age 30-64. When the HOME trial was conducted, we had to advise women to get Pap screening even if their completed home HPV test had normal results. That probably lessened their incentive to do home testing, lowered its uptake, and hindered achieving statistical significance.
Tara Beatty: HOME was an incredibly complex project with many challenges and setbacks. It was the hardworking and dedicated KPWHRI and care-delivery-system staff that made it successful.
Dr. Thayer: We adapted the electronic medical record and made relevant information on the study design available to providers viewing the results, so they could easily interpret the test in the context of the trial.
Dr. Buist: The ability to make these types of system changes to support pragmatic trials is a huge strength of Kaiser Permanente and speaks to our commitment to supporting patient safety and our clinical teams when we test innovations in health care.
More information
Here’s a UW news release about the National Cancer Institute-funded trial’s results: “Self-sampling kits helped more women get screened for cervical cancer.”
What’s next?
Dr. Buist: We’re hoping that home HPV screening could become a useful option for all eligible patients—like Kaiser Permanente Washington mailing stool test kits for colorectal cancer screening. Now Rachel Winer and I are leading a follow-on trial that will also include women who are up-to-date with cervical cancer screening.
Dr. Winer: I’m excited to build on what we learned in the HOME trial to further evaluate how home-based HPV screening could eventually become a cervical cancer screening option for all women.
Dr. Buist: We also hope to find ways to improve uptake of home testing—and follow-up of abnormal results.
Who’s who?
- Diana S.M. Buist, PhD, is a senior investigator and director of research and strategic partnerships at KPWHRI.
- Rachel L. Winer, PhD, is a professor of epidemiology at the UW School of Public Health and an affiliate investigator at KPWHRI.
- Chris Thayer, MD, was Washington Permanente Medical Group’s medical director of clinical knowledge support during the trial and is now chief medical information officer.
- Diana Miglioretti, PhD, is a senior investigator at KPWHRI and dean’s professor of biostatistics at the University of California, Davis.
- Tara Beatty, MA, is a project manager at KPWHRI.
- Kilian Kimbel was a research specialist on the trial and is now is a research communications consultant at KPWHRI.
birnbaum award
HOME Trial Team wins 2019 Birnbaum Award
Interdisciplinary group shows that home-based HPV tests can boost cervical cancer screening for women at high risk.
preventive care

Exploring at-home cervical cancer screening
Kaiser Permanente is at the forefront of research looking at using home tests for HPV, a leading cause of cervical cancer.


